N. Jb. Miner. Mh., Jg. 1991, H. 11, 481-486, Stuttgart, November 1991
Camerolaite, Cu4Al2[HSbO4,SO4](OH)10(CO3).2H2O,
a new mineral from Cap Garonne mine, Var, FranceBy Halil SARP and Pierre PERROUD, Genève
With 2 figures and 3 tables in the text
SARP, H. & PERROUD, P.: Camerolaite, Cu4Al2[HSbO4,SO4](OH)10(CO3).2H20, a new mineral from Cap Garonne mine, Var, France. – N. Jb. Miner. Mh., 1991, H. 11, 481-486; Stuttgart 1991.
Abstract: Camerolaite, ideally Cu4Al2[HSbO4,SO4](OH)10(CO3).2H20, occurs on a specimen found in the mine of Cap Garonne, Var, France. It is associated with parnauite, cyanotrichite, malachite in a quartz gangue. The crystals, blue-green in colour, form tufts, acicular and radiated-fibrous aggregates (0.5-2mm). They are flattened on {100} and very elongated parallel to [010]. The observed form are {100} and {001}. Cleavages {100} and {001} are good. Crystals are transparent, with silky lustre and pale green streak; brittle with fibrous fracture. They are non fluorescent. A chemical analysis carried out by means of electron probe: CuO 40.56; Al203 14.54; Sb2O5 13.55; SO3 4.75 and CO2 6.26; H2O 20.0 (by CHN); total 99.66 wt.%. The mineral is monoclinic, with a = 10.765(6), b = 2.903(2), c = 12.527(8) Å; = 95.61(4)°; space group P21, or P21/m; V = 389.6(7) Å3 and Z = 1. The density is 3.1(1) (measured), 2.96 g/cm3 (calculated with M.W. = 695.4), 3.09 g/cm3 (with idealized formula). The strongest lines in the X-ray powder diffraction pattern are [dA, (hkl), Ivis.]: 5.62, (10-2), 50; 5.160, (102), 90; 4.276, (20-2), 100; 3.565, (300), 40; 2.380, (013) (105) (402), 35; 2.326, (212), 35. Camerolaite is optically biaxial positive with 2Vmeas. = 77(3)°, 2Vcalc. = 75°; refractive indices at 590nm are: α = 1.626(2), β = 1.646(2), γ = 1.682(2). Optical orientation: γ = b, α ┴ {100}; dispersion r < v strong. The new mineral camerolaite is named for Mr. MICHEL CAMEROLA.
Key words: Camerolaite, antimonate, new mineral, France (Cap Garonne).
Introduction
The sample containing camerolaite was collected by Mr. MICHEL CAMEROLA in the old copper-lead mine of Cap Garonne, near Toulon, Var, France. Mineralization occurs in triassic sandstones and conglomerates. The new mineral is associated with parnauite, cyanotrichite and malachite in a quartz gangue. The mineralogy of Cap Garonne deposit was studied by GUILLEMIN (1952) and MARI & ROSTAN (1986). We gave the name of camerolaite to honour Mr. MICHEL CAMEROLA who is an eminent mineral collector. Both the mineral and the name have been approved by the IMA-Commission on New Minerals and Mineral Names, prior to publication. Holotype is preserved in the Mineralogy Department of the Natural History Museum, Geneva, Switzerland.
Physical and optical properties
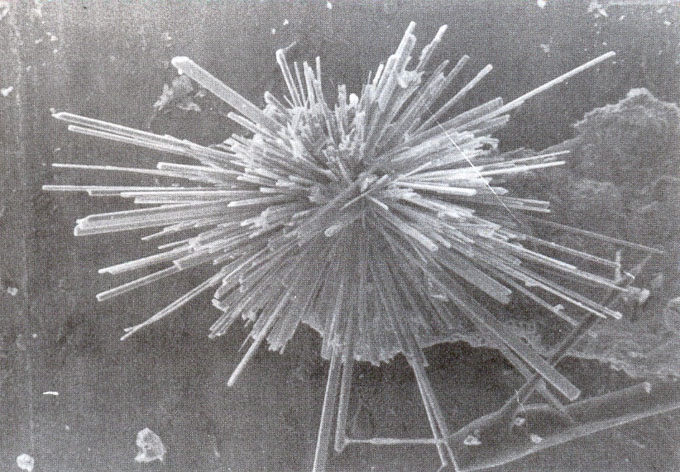
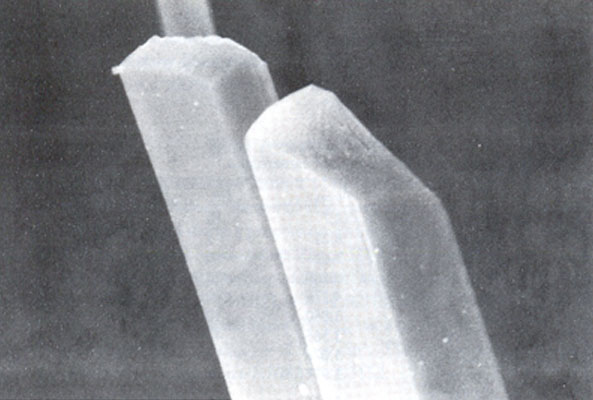
Camerolaite forms tufts and radiating fibrous aggregates (0.5-2mm) of acicular crystals (Fig. 1); these are slender and very thin (0.5 mm length and 0.01 mm width). Crystals are transparent, blue-green coloured, with silky lustre and pale-green streak. They are not fluorescent under U.V. Fracture is fibrous. Hardness could not been determined owing to the small size and brittleness of the crystals. Cleavages {100} and {001} are good. No twinning observed. Crystals are flattened on {100} and very lengthened parallel to [010]. Observed forms are {100} and {001} (Fig. 2). The mineral is soluble in HCl.
Camerolaite is optically biaxial positive with 2Vmeas. = 77(3)°, 2Vcalc. = 75°; refractive indices at 590 nm are: α = 1.626(2), β = 1.646(2), γ = 1.682(2). Optical orientation: γ = b, α ┴ {100}; dispersion r < v strong. As crystals are extremely thin, it is impossible to measure angles α /\ a and β /\ c. Pleochroism: α = colorless, β = pale-green, γ = blue-green.
The density measured using heavy liquids is 3.1(1) g/cm3. Calculated density is 2.96 g/cm3 (with M.W. = 695.4) and 3.09 g/cm3 with idealized formula. Calculation of the Gladstone-Dale relationship using constants given by MANDARINO (1981 a) gives an excellent compatibility: 1- Kp/Kc = -0.020.
Fig. 1. Tuft of camerolaite (diam. 0.6 mm). (SEM photograph, Dr. J. WUEST, Nat. Hist. Museum, Geneva.)
Fig. 2. Detail and morphology of a camerolaite crystal. The face on the left is (100); the face on the right is (001). The crystals are 0.01 mm in width. (SEM photograph, Dr. J. WUEST, Nat. Hist. Museum, Geneva.)Chemical composition
Chemical composition was studied using microprobe. Qualitative investigations showed the presence of elements Cu, Al, Sb and S. Quantitative analysis using wavelength dispersive microprobe (CAMECA) was obtained using the following standards: chalcopyrite (Cu, S), aluminum (Al), tellurantimony (Sb).
Quantitative measurements were operated under following experimental conditions: 15 kV electron-beam accelerating voltage, 2.6 nanoamperes beam current, 6 microns diameter beam. The ranges of 8 analysis and their averages are given in Table 1. H2O and CO2 were determined with HERAEUS CHN analyser.